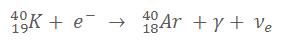Electronic capture
During an electronic capture, the nucleus of an atom captures an electron located in the inner shells. A proton of the nucleus, by absorbing this electron, becomes a neutron while emitting an electronic neutrino.
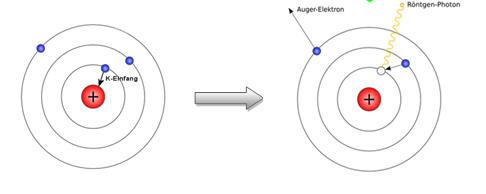
Figure 28 : Electronic capture and emission of a Gamma ray
The atom loses a proton and wins a neutron which results in this equation (example for the Potassium, That element is great ! It can decay in almost all ways):
11% of the atoms of Potassium 40 decays this way (89% in Beta- and 0.001% in Beta+). After this transformation, an Argon atom is created along with a Gamma ray with an energy of 1.46 MeV which is enough to generate a pair creation. From those 1.46 MeV, 0.51 are used to create an electron, 0.51 to create a positron and a remaining 0.22 MeV for each particle which are transferred into kinetic energy.
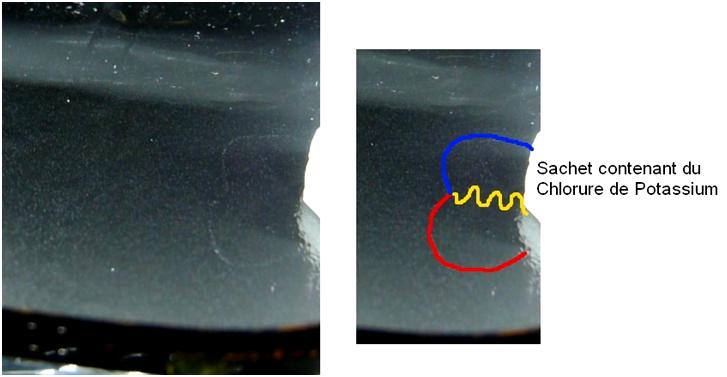
We can observe the result of such pair creation in the next image (with Gamma in yellow, electron in blue and positron in red)
Figure 29 : Pair creation resulting from the Gamma ray created in the electronic capture of one atom of Potassium 40
This figure is the proof of the transformation of one atom of Potassium 40 in Argon 40 by electronic capture with the emission of a Gamma ray .